anomalous electronic configuration|7.4: Electron Configurations of Ions : Manila 6.9: Electron Configurations and the Periodic Table "The telephone interrrupts this thought which I should never have been able to complete." Henry Miller, Tropic of Cancer (1934) "A glance at that dark, unstitched wound and a deep fissure in my brain opens up: all the images and memories that had been laboriously or absent-mindedly assorted, labelled, documented, files, sealed and stamped break forth .
PH0 · Why do the electron configurations of chromium and copper
PH1 · Why do elements in columns 6 and 11 assume 'abnormal' electron
PH2 · Second transition series (or) 4d
PH3 · Electron Configuration Anomalies
PH4 · Chromium's anomalous configuration
PH5 · Anomalous electronic configurations
PH6 · An Anomalous Electron Configuration Among 3d
PH7 · 7.4: Electron Configurations of Ions
PH8 · 6.9: Electron Configurations and the Periodic Table
PH9 · 5.12: Anomalous Electron Configurations
Find and book deals on the best cheap hotels in Glendale Heights, United States of America! Explore guest reviews and book the perfect cheap hotel for your trip.
anomalous electronic configuration*******6.9: Electron Configurations and the Periodic Table
Second transition series (or) 4d-series - simply.scienceAnomalies - An Introduction to ChemistryAnomalous electronic configurations - Chemistry Stack Exchange Cu has an anomalous electron configuration. $\ce{Cu ~=~ 1s^2~2s^2~2p^6~3s^2~3p^6~4s^1~3d^{10}}$, it does not follow the usual pattern. In this case, the 3d subshell is filled before the 4s, which usually happens in the reverse order .
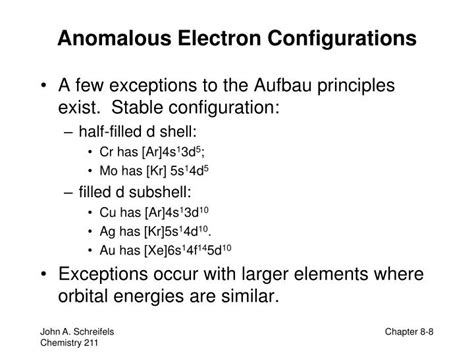
Electron Configuration Anomalies Some of the elements have electron configurations that differ slightly from what our general procedure would lead us to predict. Because a . The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis .The electron configurations of the elements indicated in blue are also anomalous, but the reasons for the observed configurations are more complex. For elements after No, the electron configurations are tentative. The expected electronic configuration (as we blindly fill the $\mathrm{d}$-orbitals along the period) is $\ce{[Ar]}\mathrm{3d^9 4s^2}$, whereas the real .Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. Define paramagnetism and diamagnetism. Justify the . It should be the first time to report such a special electron configuration in a transition metal compound, in which 4s rather than 3d orbital is preferred. Our findings reveal the distinct behavior of Sc and .
The elements having anomalous configuration are Nb 41 , Mo 42, Ru 44, Rh 45, Pd 46 and Ag 47 (six elements). These anomalous configurations are explained on the basis of . The actual electron configurations are: Cr = [Ar] 4s1 3d5. Cu = [Ar] 4s1 3d10. To understand why this occurs, it is important to realize that. 1. Completely filled .
The fourth idea in our series is that chromium displays an anomalous configuration – [Ar] 3d 5 4s 1 – because of the stability of its half-filled sub-shell. Many . The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the 9th column of the transition metal or d block. Therefore th electron configuration for silver must end as #4d^9#, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^9# This .The elements having anomalous configuration are Nb 41, Mo 42, Ru 44, Rh 45, Pd 46 and Ag 47 (six elements). These anomalous configurations are explained on the basis of nuclear–electron and electron–electron forces existing in these atoms. Complete and valence–shell electronic configuration of the atoms of 4d–series elements can be . Hence, Palladium shows anomalous electronic configuration. Share. Cite. Improve this answer. Follow answered Sep 20, 2015 at 15:35. Soundarya Soundarya. 55 3 3 bronze badges $\endgroup$ 3. 1 $\begingroup$ For 5s isn't the n+l value 5+0=5? As the l value of s subshell is 0? $\endgroup$
Molybdenum is an atom that has an anomalous electron configuration.a) Write out the expected electron configuration.b) Write out the actual electron configur.
7.4: Electron Configurations of Ions This explains the anomalous electron configuration of the transition metals and allows us to refine the electron configuration of Cu as: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 10 (paramagnetic, 1 unpaired electron) and so becomes Cu +: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 (diamagnetic; no unpaired electrons) so that we are consistent with experimental data.
Why are some electron configurations 'anomalous'? There are many example where the Aufbau principle appears to be disobeyed. For example, the ground state electron configuration of Cu is [Ar]4s 1 3d 10 , and not [Ar]4s 2 3d 9 as might be expected based on its position on the periodic table.
This chemistry video tutorial covers exceptions in electron configuration using the examples of Chromium and Copper.Electron Configuration - Introduction: . Filling Transition Metal Orbitals. The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing the electron configuration for the other rows of transition metals. The noble gas before the .
anomalous electronic configuration Note: The unabbreviated electron configuration of palladium is [Kr] 4d 10. When writing an electron configuration, you have to write serially. Palladium ion(Pd 2+) electron configuration. The ground state electron configuration of palladium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This electron configuration shows that the d . Cu has an anomalous electron configuration. Cu = 1s2 2s2 2p6 3s2 3p6 4s1 3d10, it does not follow the usual pattern. In this case, the 3d subshell is filled before the 4s, which usually happens in .We would like to show you a description here but the site won’t allow us.
Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written (here is an explanation why). Therefore we have (still incorrect) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2. Correct Electron Configuration for Copper (Cu)
anomalous electronic configuration 7.4: Electron Configurations of Ions Some elements do not follow the Aufbau principle, there are some alternate ways that electrons can arrange themselves that give these elements better stability. Using the Aufbau principle, you would write the following electron configurations Cr = [Ar] 4s^2 3d^4 Cu = [Ar] 4s^2 3d^9 The actual electron configurations are: Cr = [Ar] 4s^1 3d^5 . In the second-row transition metals, electron–electron repulsions within the 4d subshell cause additional irregularities in electron configurations that are not easily predicted. For example, Nb and Tc, with atomic numbers 41 and 43, both have a half-filled 5s subshell, with 5s 1 4d 4 and 5s 1 4d 6 valence electron configurations . The initial configuration is : [Xe] 4f 14 5d 8 6s 2, then one electron is transfered from 6s to 5d, so that all orbitals become stable, either through full filling or half filling, which is better then having one empty and unstable. The question of anomalous electronic configurations, meaning $\mathrm{s^1}$ or $\mathrm{s^0}$ in one case (Pd) is very badly explained in textbooks. For example, the anomalous configuration of Cr ($\mathrm{3d^5~4s^1}$) is typically explained as being due to "half-filled subshell stability". This is wrong for several reasons.

Anomalous electronic configuration in the 3d series are of (a) Cr and Fe (b) Cu and Zn (c) Fe and Cu (d) Cr and Cu. Answer. Answer: d. . General electronic configuration of actionoids is (n – 2)f 1 – 14 (n – 1)d 0 – 2 ns².Which of the following actinoids have one electron in 6d orbital? [NCERT Exemplar]Therefore, the electronic configuration of sulfur can be written as 1s 2 2s 2 2p 6 3s 2 3p 4. The electronic configuration of elements can also be written with the help of noble gases. These noble gases have completely filled outermost shells and can be prefixed to the outermost shell of the element whose electronic configuration must be noted.
Ohne Einzahlung Exklusiver Bonus für alle neuen Spieler im LevelUp Casino: 15 Freispiele gratis nach Registrierung für "Cleopatra's Gems" Umsatzbedingungen Bonus ohne Einzahlung: Spielautomaten - 70x Gewinne aus Freispielen Einzahlungsbonus Einzahlung: 130% bis zu 100€ + 100 FS mit dem Code: LVLUP130 Einzahlung: 50% bis 100€ + 50 .
anomalous electronic configuration|7.4: Electron Configurations of Ions